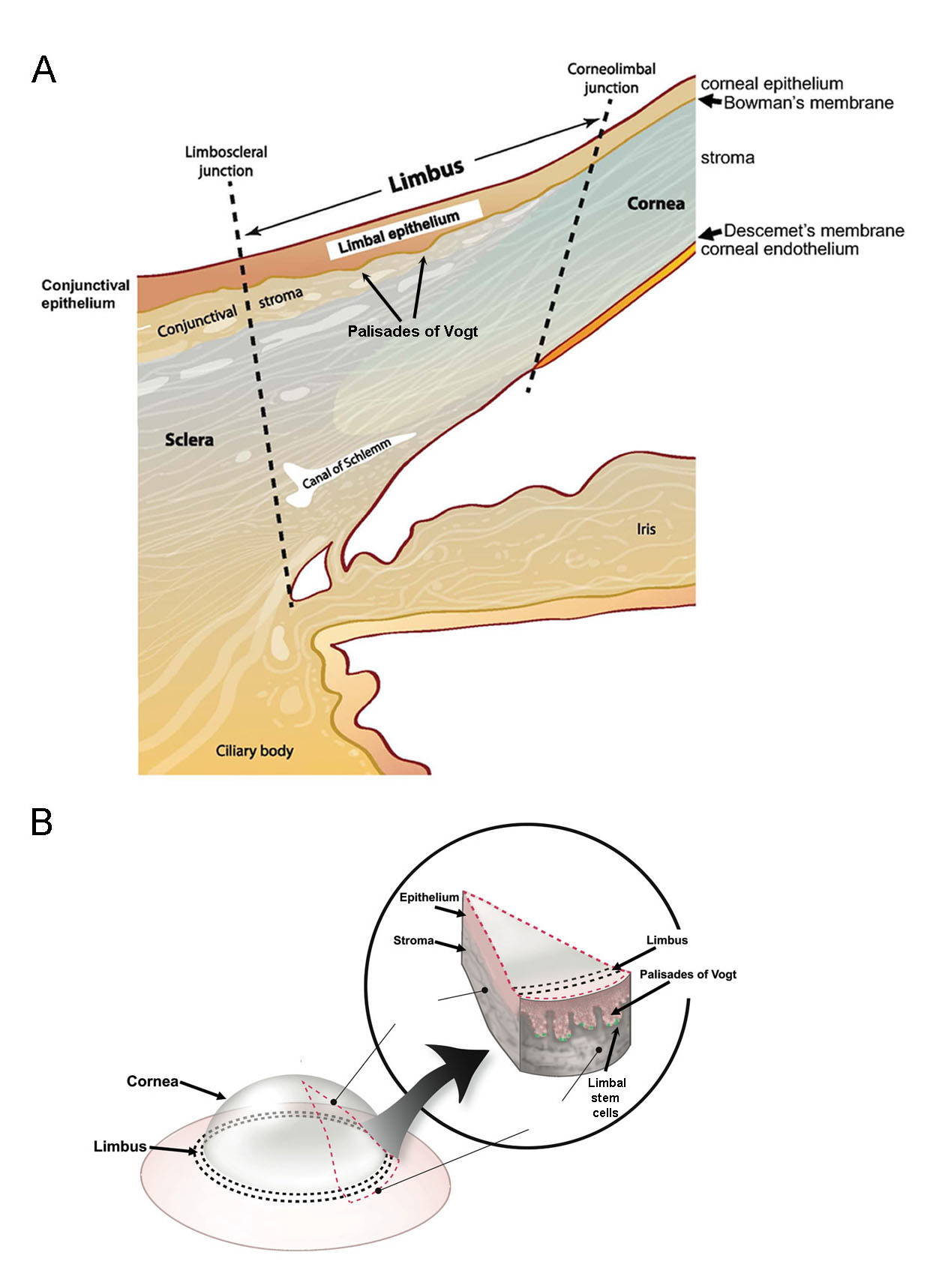
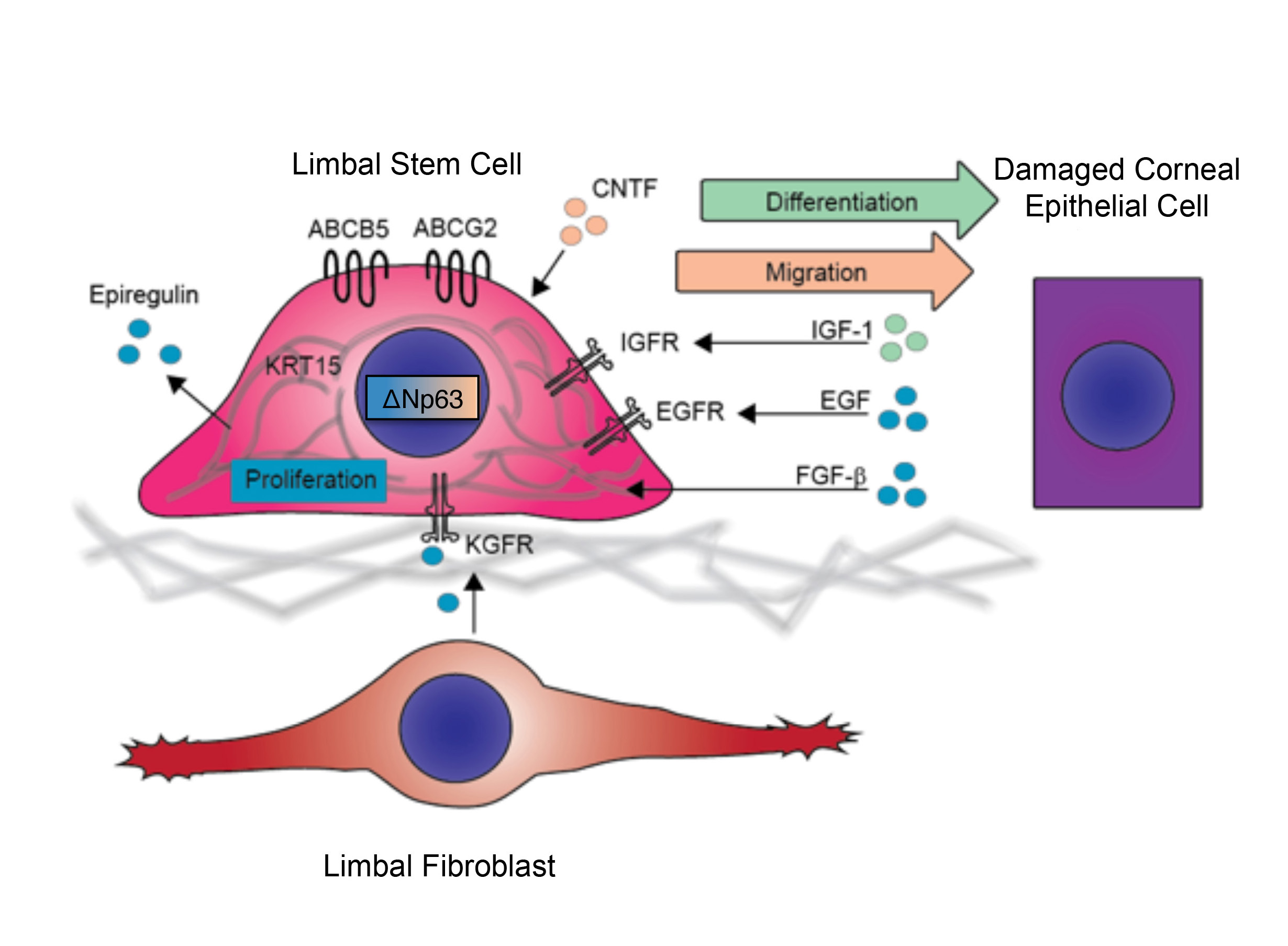
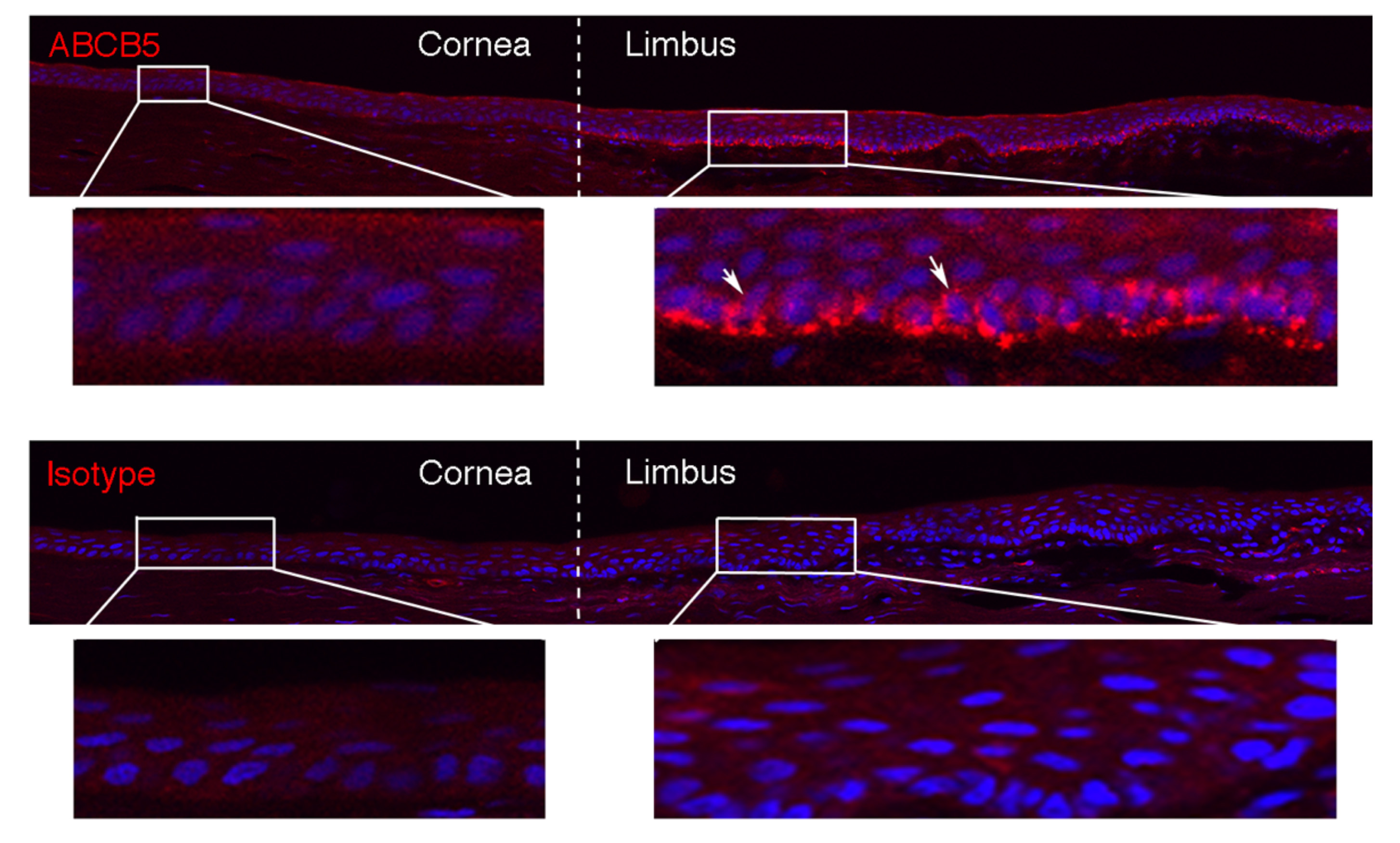
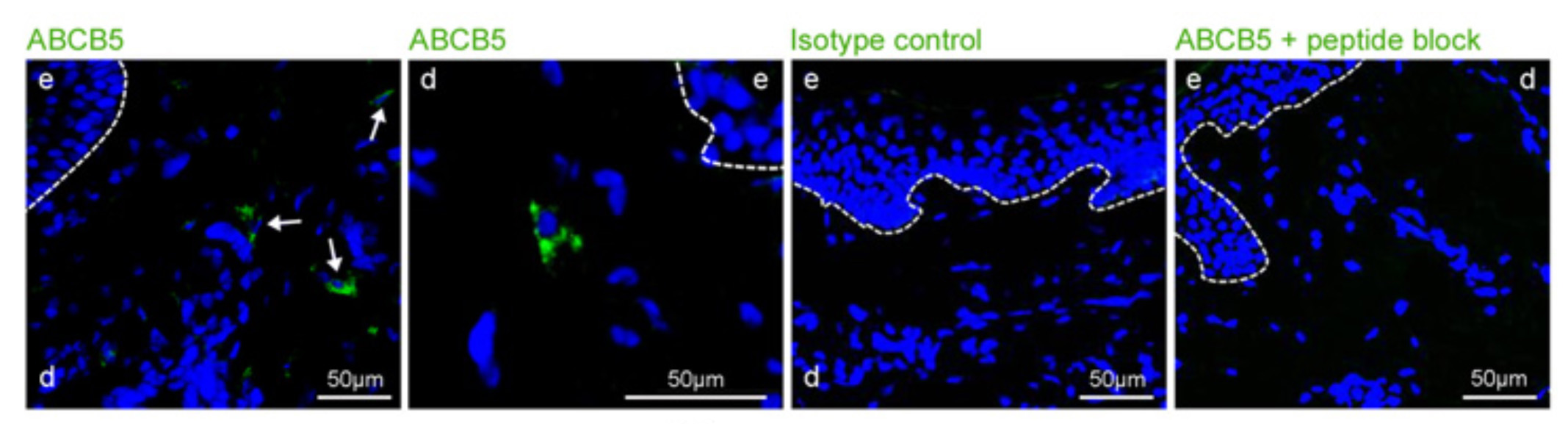
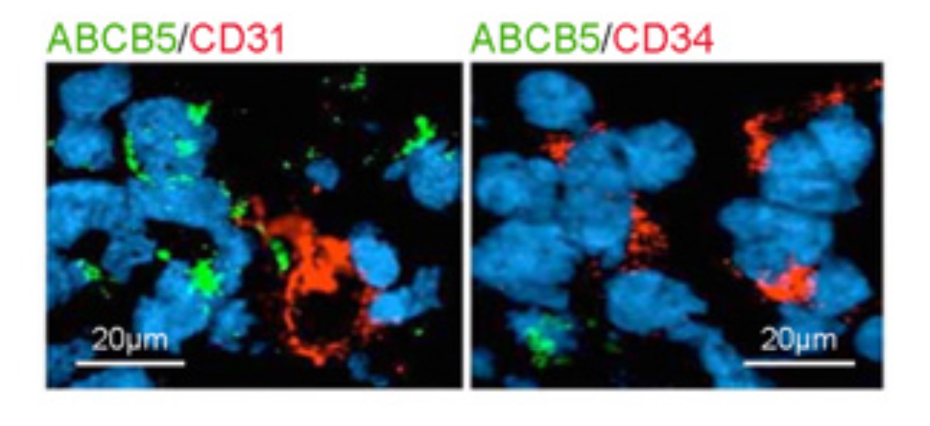
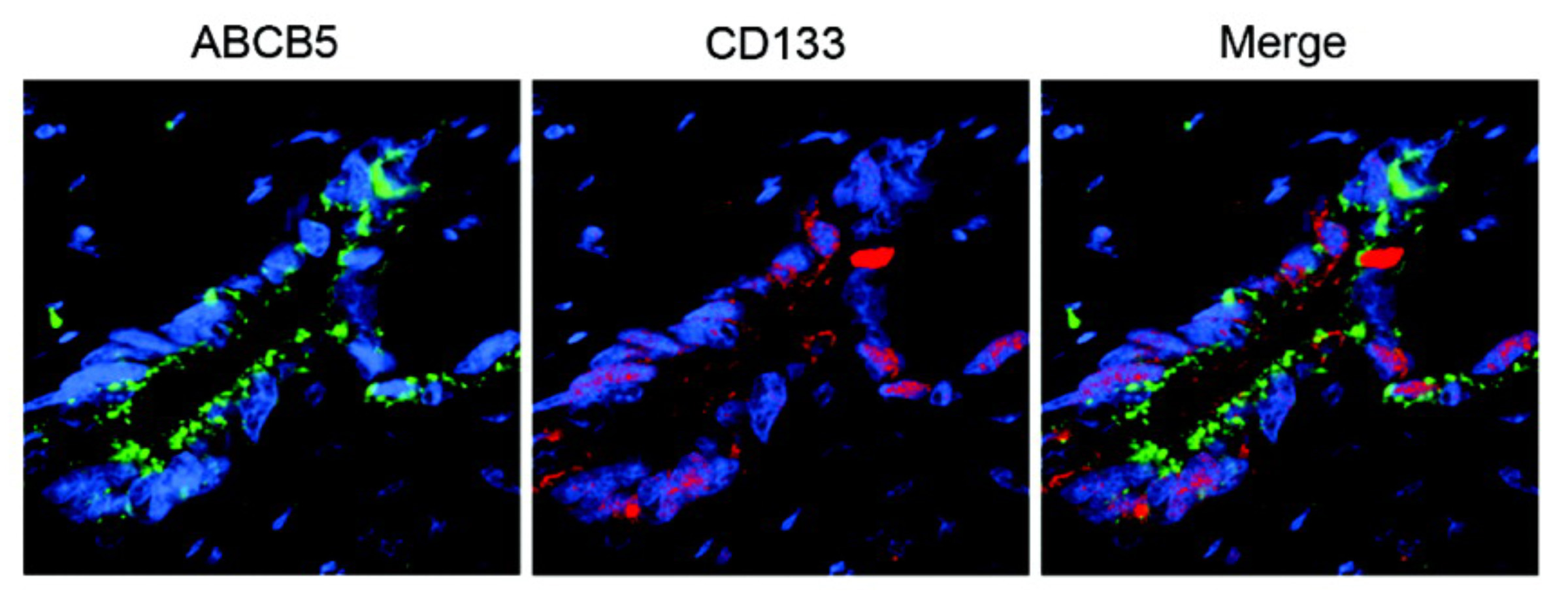
Our laboratory cloned and characterized the novel ATP-binding cassette transporter, ABCB5, and showed that this plasma membrane-expressed P-glycoprotein homologue functions in self-renewal and maintenance of slow-cycling adult stem cells. In the mammalian eye, ABCB5 is essential for corneal development and repair through regulation of limbal stem cell (LSC) maintenance and survival. Importantly, with our collaborators, we also showed that purified ABCB5-positive LSC grafts are capable of fully restoring the cornea in preclinical models of limbal stem cell deficiency (LSCD), providing a clear rationale for clinical transplantation trials involving this stem cell population to improve therapy for LSCD and potentially additional corneal disorders.
Wound healing is a highly regulated process involving cell migration, proliferation and secretion of extracellular matrix proteins, which is critically dependent on the intact function of stem cells residing in diverse tissues. Our studies utilizing Abcb5 knock out (KO) mice revealed a critical functional role of ABCB5 in normal cutaneous wound healing, based on the observed delayed wound closure, increased inflammatory stroma thickness and aberrant angiogenesis in the KO animals. Transplantation of human ABCB5+ human dermal stem cells resulted in significant improvement of wound healing in preclinical studies in mice. These findings led to several clinical trials investigating this approach for the treatment of chronic wounds in human patients.
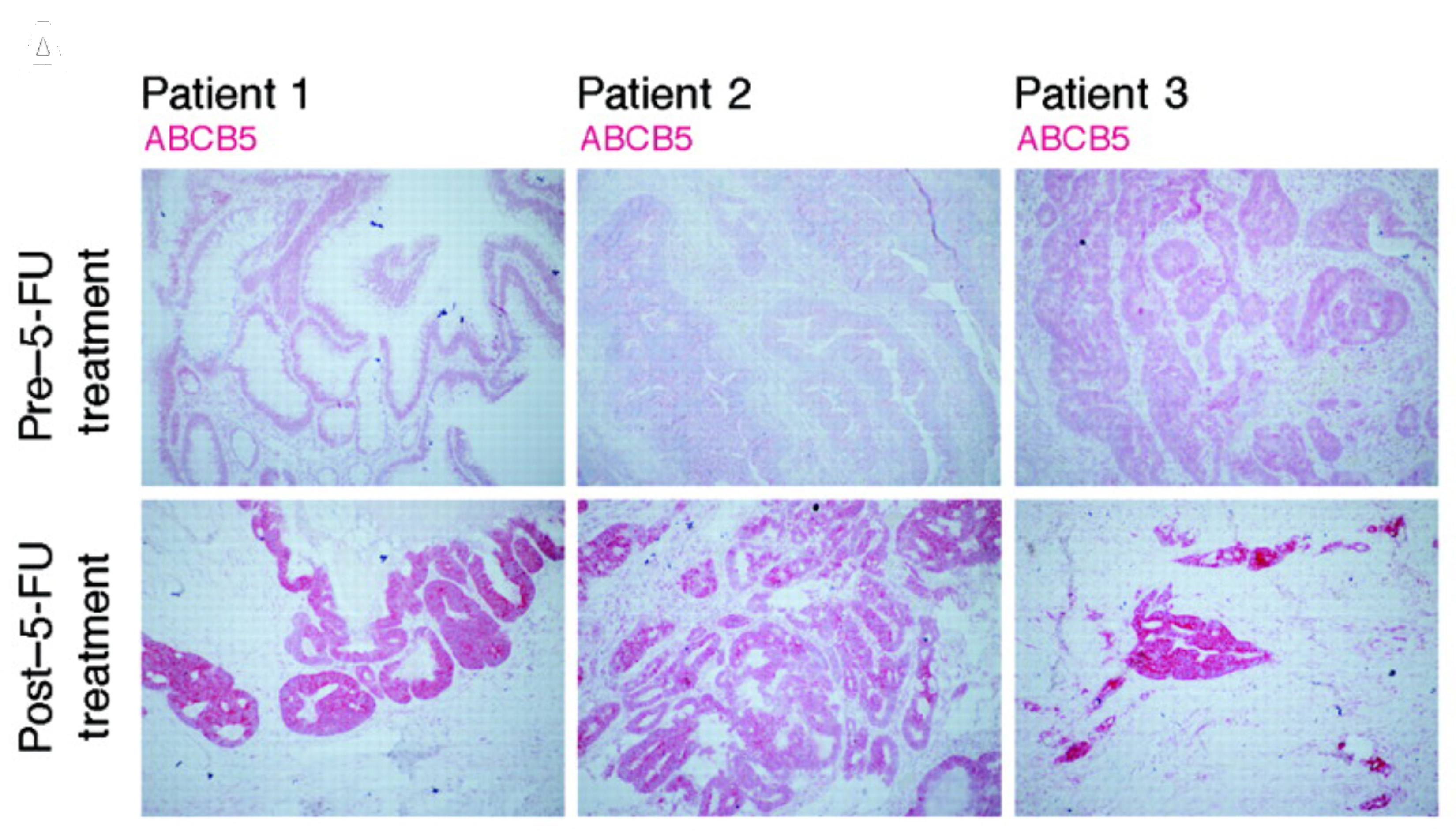
In human cancer, ABCB5 has also been shown to control stem cell maintenance and to be responsible for cancer stem cell-driven tumor initiation, drug resistance and metastatic disease progression. Specifically, our laboratory established that ABCB5 confers clinical drug resistance to the first-line chemotherapeutic agent, 5-fluoruracil, on colorectal cancer stem cells; as a result, we now seek to further dissect ABCB5 functions in colorectal cancer stem cells and to target newly discovered ABCB5 anti-apoptotic functions in this malignancy to ultimately improve clinical colorectal cancer therapy.
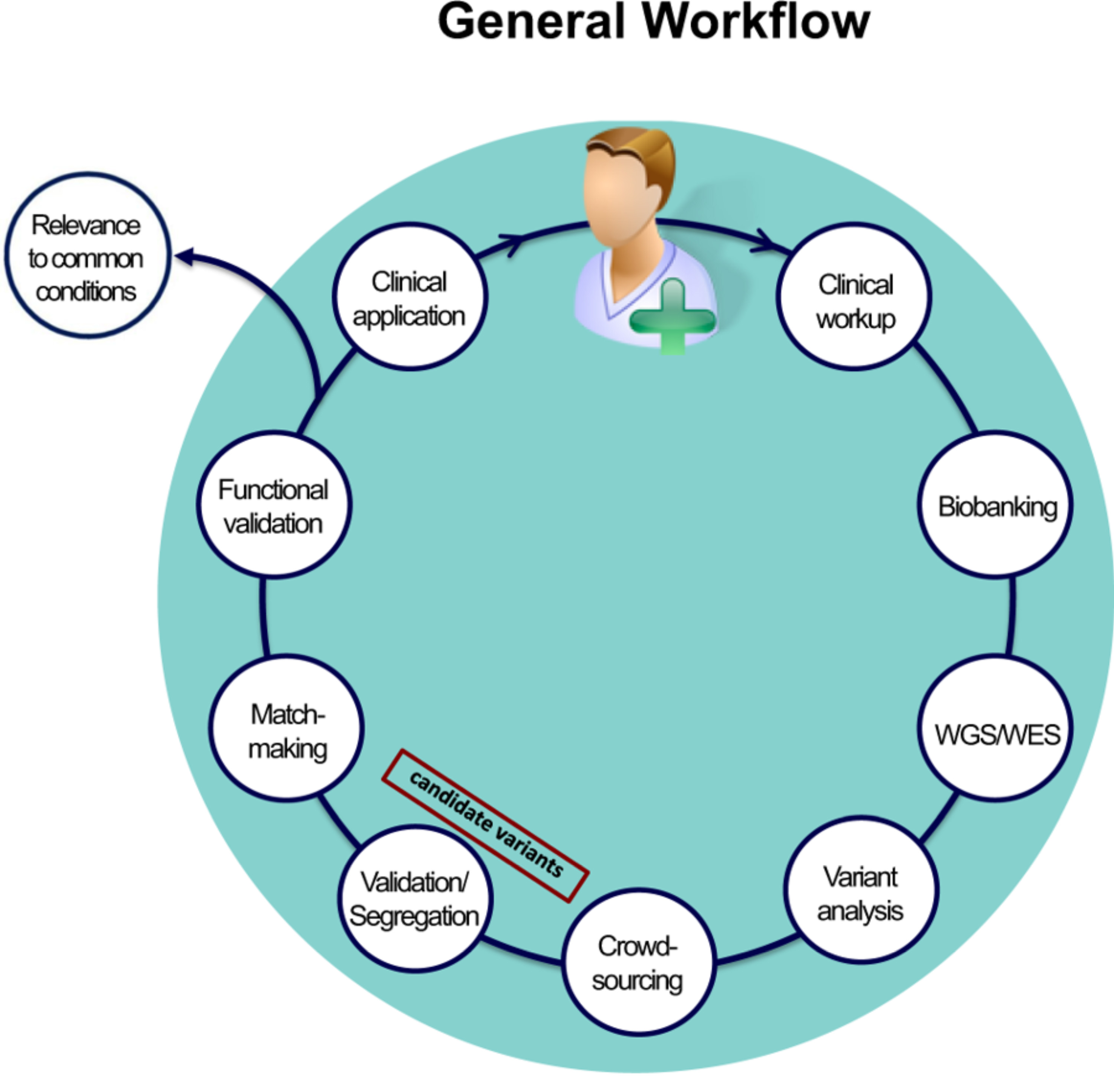
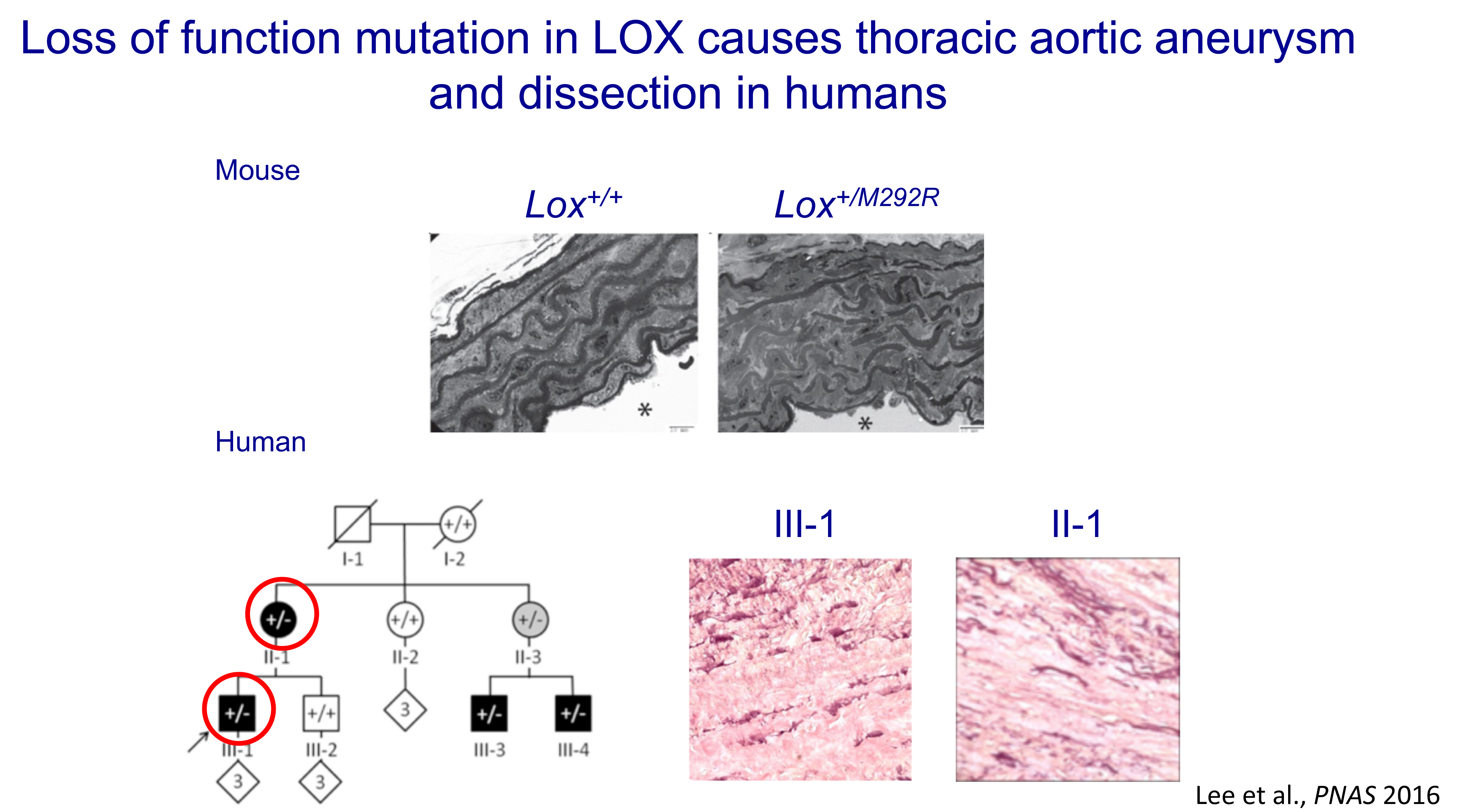
We are members of the Brigham Genomic Medicine (BGM), which employs the unique genomic analysis pipeline which won the international 2012 CLARITY Challenge to solve undiagnosed cases, even when prior whole genome sequencing (WGS) analyses have been unrevealing. In our most recent novel clinical cases, WGS followed by experimental validation led to successful genetic diagnosis with important implications for patient care.